Human genetics and drug development
Drug development is a risky business. Less than 1 out of 10 drugs that enter clinical development succeeds (success defined as getting an FDA approval and entering the market). Because of this extremely low success rate, the cost of developing a drug has skyrocketed. The expectation is that to land on one successful drug, you should work on at least 10, to increase your odds of succeeding. And landing on that one success will offset all the money wasted in developing the failed drugs. This current business model demands huge investment to run a drug business, often in the range of billions of dollars. In a such a scenario, strategies that can push the base success rate beyond 10% will prove highly valuable for drug companies. One such strategy is to embrace human genetics.
Recently I contributed a 'Views and News' article to GEN Biotechnology journal on invitation from the journal's executive editor Kevin Davies. The commentary was on an article published recently in Nature by Matt Nelson and colleagues on the value of human genetics to improve drug success.

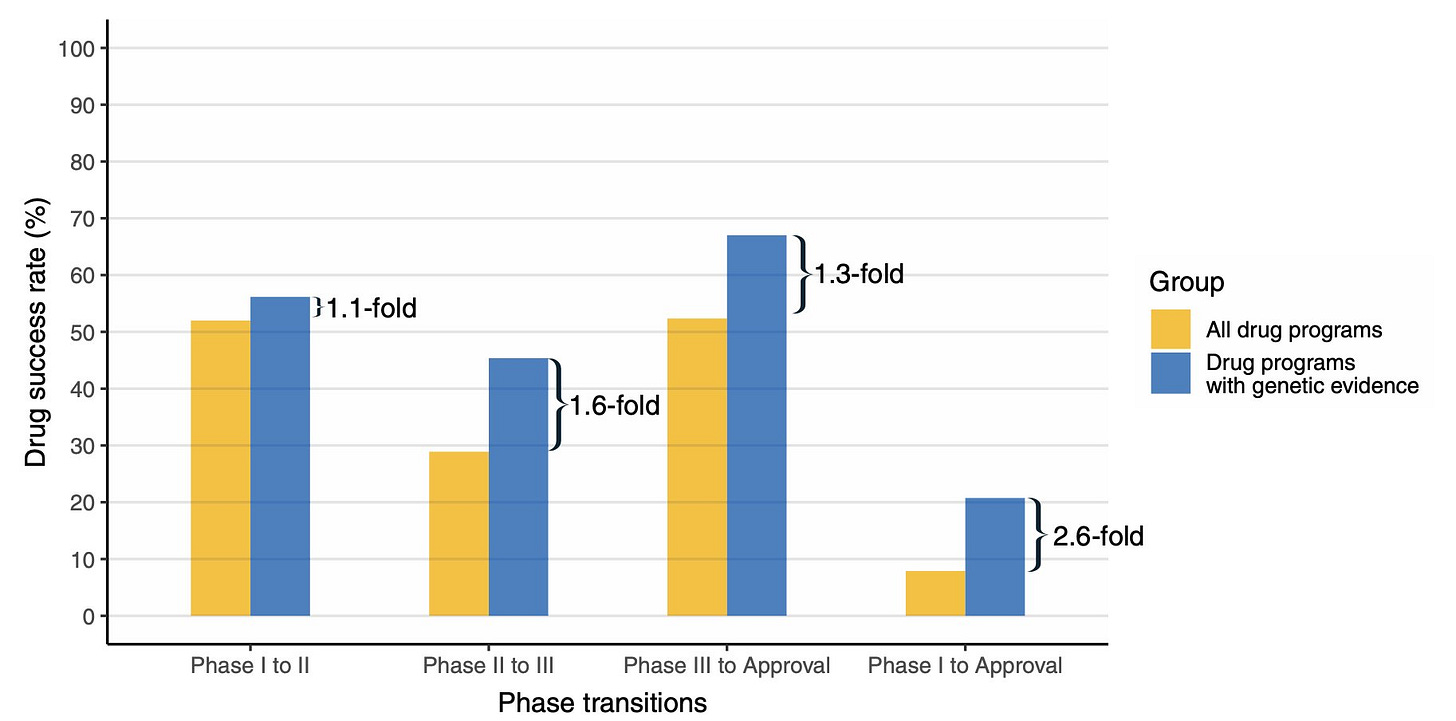
Nelson is well known in the field for his landmark article in Nature Genetics from 2015. It was the first empirical demonstration that human genetic evidence can increase the odds of drug success. Through a systematic analysis of public and proprietary databases, Nelson et al. arrived at the conclusion that prioritizing drug targets based on human genetics evidence can nearly double the success rate. The paper was a hit. The impact it had on both industry and academia was beyond Nelson's or anyone's expectation. It became an essential reference in investment pitches made to biotech VCs. It has probably influenced many big investment decisions in the past decade.

Nelson and colleagues' recent work is a follow-up of their 2015 analysis. The core idea of the analysis in both their articles is to curate a list of drug programs that are in various stages of development and evaluate how often programs backed by human genetics succeeded compared to others. It's important to understand what, according to the authors, qualifies as a "human genetics evidence". If the gene corresponding to the drug (that is, the drug works by targeting either the gene or its product) has any evidence of association with the indication in the literature, it is considered to have a human
...This excerpt is provided for preview purposes. Full article content is available on the original publication.