Why Capturing Carbon from the Air Will Always Be Expensive

If you’ve been following the climate crisis, you’ve probably heard that we need to get to zero carbon emissions. Quite simply: the more CO₂ there is in the air, the more the planet warms, and so the only way to stop the warming is to stop emitting carbon.
This is a difficult task. It’s so difficult that many experts believe we won’t get to zero without some form of ‘negative carbon emissions’, and one big idea here is to develop new technologies that suck carbon dioxide out of the air on a global scale.
But any future technology extracting carbon from the air will never be a substitute for reducing our carbon emissions. That’s because there’s a basic cost to capturing carbon dioxide — a cost imposed by the laws of physics — and the bad news is, it isn’t cheap.
Why Extracting CO₂ From The Air is Inherently Inefficient
Let’s say you come across some advanced future technology that sucks CO₂ molecules out of the air (perhaps handed to you by an environmentally conscious time-traveler).
Surprisingly, even if we know nothing about how this machine works, we can still use physics to work out the minimum amount of energy that it consumes. All we need to know is what the machine does, and what it does is trap CO₂ molecules in a compartment (like a vacuum cleaner, but for CO₂).
Here’s a cartoon of what the air in your room looks like before you turn on the machine (the red dots represent CO₂ molecules).
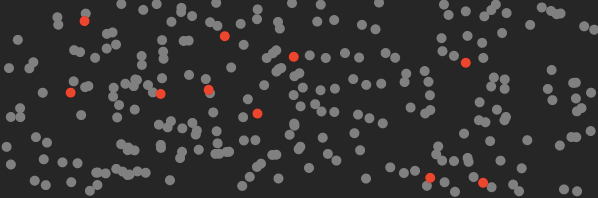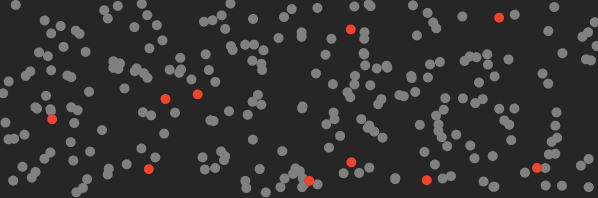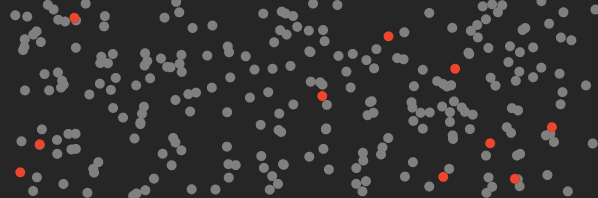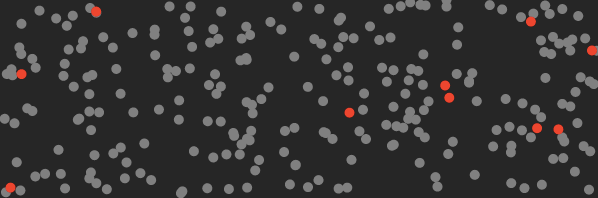
And here’s what it looks like after this machine traps the CO₂.
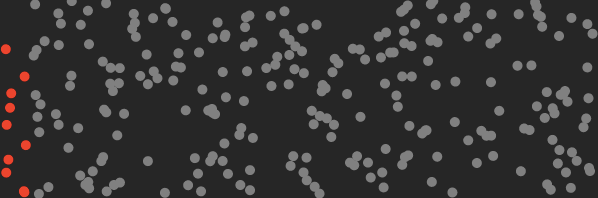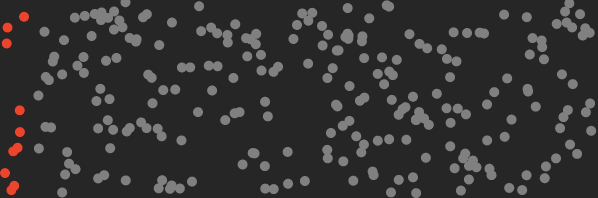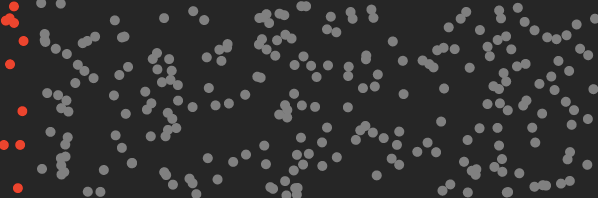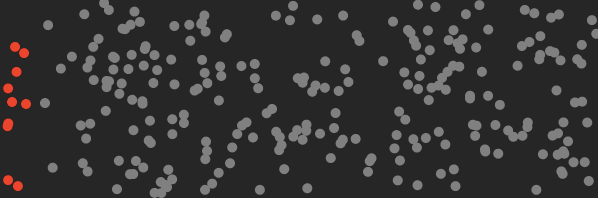
By separating CO₂ molecules, the machine reduces the entropy of the air by an amount that can be calculated.
Why Does This Machine Decrease The Air’s Entropy?
Roughly speaking, entropy measures the disorder of the air molecules. By separating the CO₂ we’ve arranged the molecules in a more orderly state, i.e. we’ve decreased its entropy.
You might think this entropy change is impossible to calculate without knowing more about the machine. But the air’s change in entropy depends on what happened, not how it happened. And since we know the initial and final state of the air — mixed vs. separated — we can calculate its change in entropy.
But the second law of thermodynamics teaches us that if you reduce entropy somewhere,
...This excerpt is provided for preview purposes. Full article content is available on the original publication.