DeSci in Action: VitaRNA Tokenized Gene Therapy Achieves Key Safety Milestone in First Animal Studie
VitaRNA has released first in vivo data showing their lead gene therapy candidate, ARTAN-102, is safe and reaches multiple tissues in mice. The 14-day study marks an important preclinical milestone for one of the first tokenized gene therapy projects.
Led by CEO Anthony Schwartz and CSO Michael Torres - top-tier biotech executives with a proven track record of advancing drug development platforms into human clinical trials - the project has progressed from initial VitaDAO funding ($91k) and registration onchain as an IP-NFT, through a subsequent $300k raised via IP tokenization, to reach several key milestones in developing tRNA suppressors targeting nonsense mutations.
Study Results
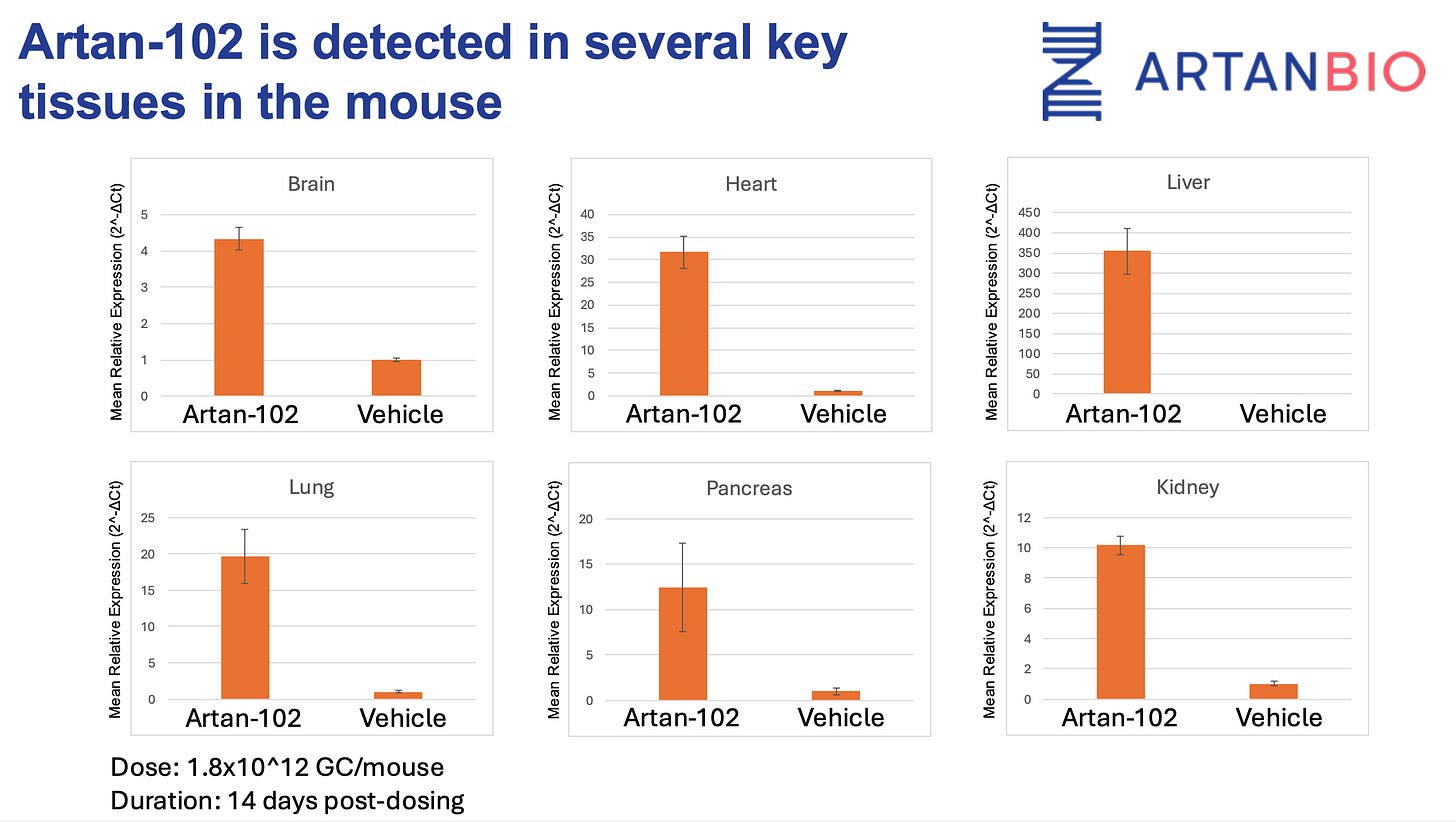
The mouse study (n=4 per group) evaluated ARTAN-102 delivered at 1.8×10¹² GC/mouse and found:
No changes in body weight or blood cell counts
No adverse events
Successful delivery to all six tissues examined, with highest expression in liver (350-fold increase), followed by heart (32-fold), lung (20-fold), pancreas (12-fold), kidney (10-fold), and brain (4-fold)
The Science
ARTAN-102 targets arginine nonsense mutations (CGA codons) that accumulate with age and disrupt proteins like p53, ATM, and APP. These mutations contribute to aging and various diseases. The therapy uses AAV9 to deliver genetic suppressors that restore normal protein function.
Supporting research published in Nature Aging (January 2025) shows CpG mutations accumulate over time and correlate with epigenetic aging, validating the therapeutic approach.
Progress Update
Initial screening identified 2 lead candidates from 15 vectors
Filed 2 provisional patent applications
Manufacturing partnership established with Lonza for GMP production
Currently raising $500k for non-human primate studies
Targeting $5M total to reach human trials within 12 months
The project has progressed from VitaDAO's initial $91k funding and $300k IPT raise to achieving pharmaceutical-grade manufacturing and safety data.
What This Means
This data demonstrates that DeSci-funded projects can produce legitimate therapeutic candidates. By combining community funding with traditional drug development, VitaRNA has achieved in months what typically takes years in conventional biotech.
Next steps include completing primate toxicology studies and finalizing GMP manufacturing before pursuing first-in-human trials.
Dataroom: https://molecule.xyz/ipts/VITARNA?shareFileDid=did:odf:fed01f889955f203b0d5383ff64fc1c6e1ef6c9b16613925f8edf5effda4e4ef110eb
Learn more: https://vitarna.xyz
Join the community: https://t.me/vitarna
Coingecko: https://www.coingecko.com/en/coins/vitarna
This excerpt is provided for preview purposes. Full article content is available on the original publication.